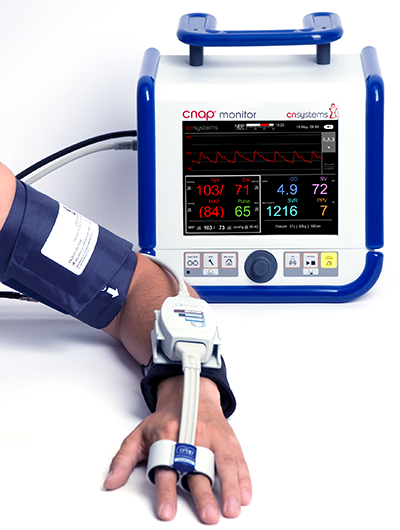
CNAP Monitor 500 "HD"
Continuous Noninvasive Blood Pressure & Hemodynamics
- Product No: NS-1066
- Manufacturer: CNSystems
- PLETH
- Request Quote
Description
The new CNAP Monitor 500 ‘HD’ is a non-invasive and beat-to-beat system which records blood pressure, hemodynamic (CO, SV etc.) and fluid status (PPV/SVV) with the same accuracy as invasive systems. The CNAP Monitor solution is trending rapidly thanks to its reliability, cost-effectiveness and fast set-up requiring minimal training effort. The blood pressure curve is available immediately after application. The new CNAP Monitor 500 ‘HD’ has also expanded limits of application, supporting the implementation of Enhanced Surgical Recovery (ESR) with Goal Directed Therapy (GDT) in low and intermediate risk surgeries (also in parallel to the arterial line without the need for an arterial catheter) in ER and bariatric patients. In research applications, CNAP HD enhances standard hemodynamic assessment with continuous blood pressure and full hemodynamic from an easy-to-use finger sensor.
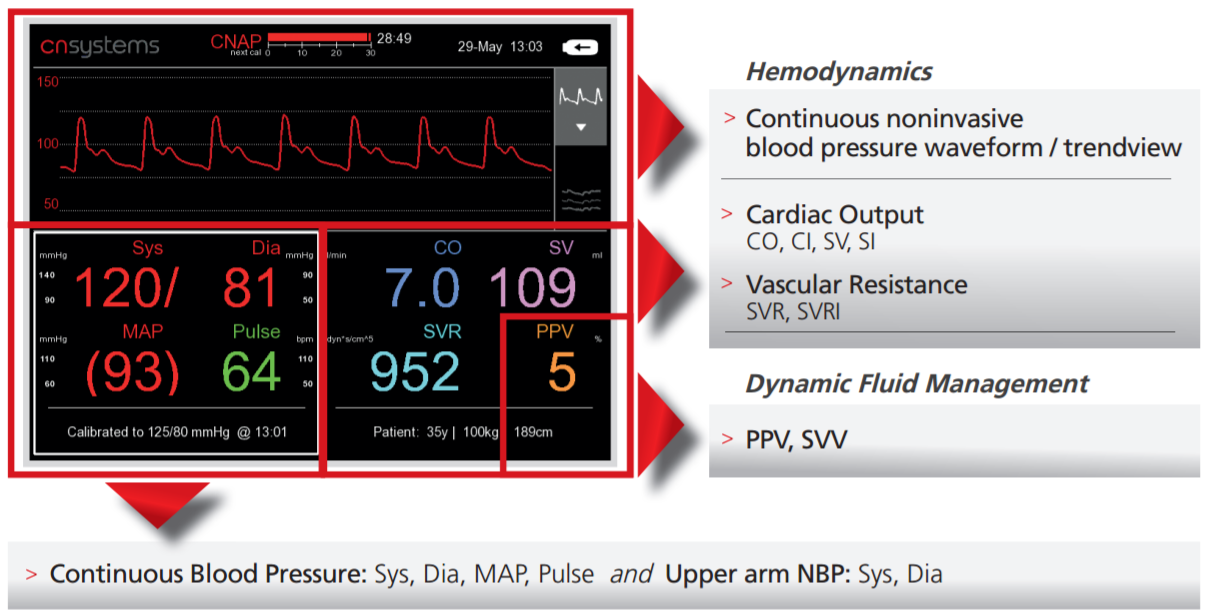
Full Hemodynamic Picture
System Features
- Real-time systolic, diastolic, mean blood pressure and pulse rate
- High-fidelity blood pressure waveform
- CO (Cardiac Output with CNAP® HD and Pulse Contour analysis), SV (Stroke Volume), PPV/SVV (Pulse Pressure Variation, Stroke Volume Variation)* and SVR (Systemic Vascular Resistance) allowing for hemodynamic monitoring and fluid optimization*
- Easy-to-use double finger sensor for a quick and easy setup for every day clinical use
- Automatic sensor sizing
- Different calibration modes to upper arm blood pressure (NBP, oscillometric)
- Easy integration in patient monitoring "BP Wave Out"
- Adjustable alarming system
- 8.4” colour anti-reflective screen
- Integrated thermal printer
- Rechargeable internal battery for up to two hours of CNAP® operation
- Up to 24 hours measurement duration
- Additional analog out connector - customizable assignment
Technical Specifications
| Continuous Noninvasive Arterial Pressure | |
|---|---|
| Sys | 40 - 250mmHg |
| Dia | 30 - 210mmHg |
| Mean | 35 - 230mmHg |
| Pulse rate | 30 - 200bpm |
| Degree of protection | BF (defibrillation proof) |
| Automatic scaling to brachial pressure (NBP) | |
| Oscillometric Blood Pressure Measuring Rage | |
| Dia | Adult 20 - 200 mmHg, Pediatric 20 - 160 mmHg |
| Sys | Adult 40 - 260 mmHg, Pediatric 40 - 230 mmHg |
| Degree of Protection | BF (defibrillation proof) |
| CNAP Hemodynamics Measuring Range | |
| CO | 0,0 - 99,9 l/min |
| CI | 0,0 - 99,9 l/min/m2 |
| SV | 0 - 500 ml |
| SVI | 0 - 500 ml/m2 |
| SVR | 0 - 9999 dyne*s/cm5 |
| SVRI | 0 - 9999 dyne*s/cm5 /m2 |
| Fluid responsiveness: CNAP PPV and SVV | |
| Measuring range | PPV 0 - 40% |
| SVV 0 - 40% | |
| Electrical | |
| Nominal voltage | 100 - 240 VAC |
| Supply frequency | ~50/60 Hz |
| Battery | sealed lead-gel, operating time: 2 hours (fully charged battery) |
| CNAP Hardware | |
| Weight | 7,5 kg (16,6 lbs) including accessories and cables |
| Height | 280 x 270 x 250 mm (11 x 10,6 x 9,8 inch) |
| Built-In Monitor | |
| Type | TFT-LCD, 800 x 600 pixel |
| Size | 8,4 inch diagonally |
| User Interface | |
| Controls | Click-wheel control, fast access keys |
| Indicators | Visual and audible alarm indication, battery status, printer status, power LED |
| Trend Display | Customized configuration: numeric, graphic, alarm history |
| Adjustable Alarming System | |
| Alarms | physiological: med priority, technical: low priority |
| Connectivity | |
| BP Wave Out | Easy integration in all standard patient monitoring systems (2 - 10 VDC supply voltage) |
| AUX Analog Out | Analog output of calibrated continuous blood pressure waveform (-5V to 5V) |
| USB Port | |
| Version | USB 1.1 (bandwidth 12 MBits/s) |
| Printer | |
| Type | Integrated thermal printer, 58mm |
| Compliance and Approvals | |
| Safety class II (IEC 60601) | IEC 60601-1; IEC 60601-1-6; EN 1060-4 (NBP) |
| Class II b (93/42/EEC) | IEC 60601-1-2; IEC 60601-1-8; ISO 81060-2 (NBP) |
| Patient applied part type BF | (defibrillation proof); IEC 80601-2-30 |
Please note: Important! This product is for research applications only. Not a medical device as defined in EU directive 93/42/EEC. Not designed or intended to be used for diagnosis or treatment of disease.